Development & Manufacture of Packaging Solutions

We are available to develop and manufacture plastic packaging solutions or Class I medical devices, providing extensive support from the initial drawing stages up to full-scale production.
With our expertise, we can provide you with prototype development and 3D-printing, mould and tooling design and manufacture, small-volume production and parts assembly.
We have state-of-the-art plastic injection machines that comply with the strict production requirements of primary pharmaceutical packaging and Class I Medical Devices.
PlasticProgress Pharma Packaging is certified to ISO 9001 and ISO 15378 standards, and we are compliant with GMP (Good Manufacturing Practices) standards.
Our certification for the production of Class I Medical Devices according to Directive 93/42/EEC, Annex V standards, is currently on hold. But we have kept all procedures in place so we can readily undergo the re-certification audit and resume full compliance to produce for you high quality Class I medical devices with CE-mark or requiring a measuring function.
As a flexible Pharma Packaging Solutions provider, we are available to manufacture from low pre-series quantities up to medium/high production volumes at competitive prices.
Production, assembly and packaging of your products is performed in our regularly audited and certified ISO Class 7 cleanroom, with HEPA filters and with a constant ISO Class 5 laminar flow over the injection area, all according to ISO 14644-1 standards.
We assure you that the development and manufacture of your primary plastic pharma packaging or Class I medical devices is in excellent hands with us.
Product types
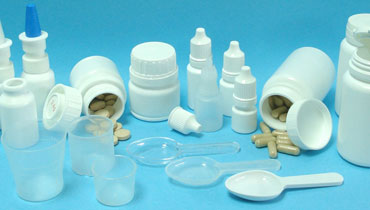
PlasticProgress Pharma Packaging manufactures a standard range of products, such as snap-on bottles, eye-droppers, container bottles for solid oral formulations and Class I medical devices such as cups and spoons.

We are available to develop and manufacture pharma plastic packaging solutions and Class I medical devices, providing extensive support from the initial drawing stages up to full-scale production.

We offer a streamlined, turn-key operation for the contract manufacturing of your own primary plastic pharma packaging, Class I medical devices and components.

